In this article (and video above), we calculate the BOD (Biochemical Oxygen Demand) of wastewater that forms part of the Civil and Environmental Engineering section of the FE exam to give you a better understanding of what you can expect during the exam. This problem was solved by Enrique Ivers, an Engineer in Training.
Question:
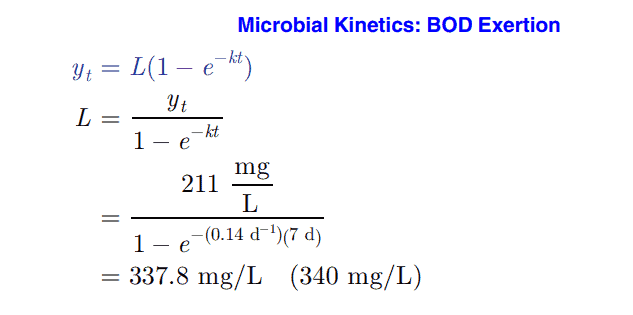
A sample of wastewater is incubated for 7 d at a temperature of 20°C. After this incubation period, the BOD is found to be 211 mg/L. The reaction rate constant is 0.14 d-1 (base e) The ultimate BOD of this sample is most nearly:
A: 225mg/L
B: 310 mg/L
C: 340 mg/L
D: 560 mg/L
Solution:
Substitute the seven-day values into the rearranged equation for BOD exertion. See the full solution in the video at the top of this post.
Answer:
The correct answer is C: 340 mg/L
This Episode Is Brought to You by PPI

I hope you found this week’s Biochemical Oxygen Demand (BOD) prep question helpful. In upcoming articles, I will answer more FE Exam questions and run through more practice problems. We publish videos bi-weekly on our Pass the FE Exam YouTube Channel. Be sure to visit our page here and click the subscribe button as you’ll get expert tips and tricks – to ensure your best success – that you can’t get anywhere else. Believe me, you won’t want to miss a single video.
Lastly, I encourage you to ask questions in the comments of the videos or here on this page and I’ll read and respond to them in future videos. So, if there’s a specific topic you want me to cover or answer, we have you covered.
I’ll see you next week.
Anthony Fasano, P.E.
Engineering Management Institute
Author of Engineer Your Own Success

Leave a Reply